Summary
Börje Svensson (2015) in his book Magic Molecule: that has improved the lives of millions" explained the discovery of hyaluronic acid which has numerous medical implications. According to him, in 1934, hyaluronic acid was first discovered by John Palmer and Karl Meyer, at the University of Colombia. However, for commercial use, hyaluronic acid was first time discovered by Endre Balaz, a Hungarian researcher. Balazs also tried to discover the commercial applications of hyaluronic acid, particularly its medical benefits. In his whole career, he was so eager to transform researches into the practical benefit and achieved so much that many researchers only can dream of.
Nowadays, hyaluronic acid is also termed as hyaluronan and is its abbreviation is HA. It is also found in different regions of human body hyaluronic acid in human skin works as the filling and moisturizing agent. Moreover, it works as a shock absorber and lubricant in human joints along with that it is found in umbilical cord and eye. It can be produced synthetically through fermentation and also originates from the rooster combs. The medical benefits of hyaluronic acid include that it makes eye lens removal and its replacement with artificial one easy. It also eases ailment of knees which are infected by arthritis among humans as well as among horses. Its benefits do not end for horses, knees, and eyes; it also plays a significant role in revolutionizing beauty industry all over the world in last two decades. It is most prominently used as a critical ingredient of dermal filler and wrinkle reducing products.
People all around the world due to high beauty standards of the society try to rejuvenate their skin as well as their facial features by the use of Restylane®, Juvéderm® and Botox® cosmetics. At the brink of this worldwide movement there is multinational pharmaceutical named Allergan which is also considered as a leader of facial injectables. Already in 2013, the facial aesthetic market had a value of around $2.5bilion, a figure which is expected to increase two-fold by 2020. The leading position of Allergan became possible by success of Botox® Cosmetics which is also known as a neuromodulator and the Juvederm® which is a dermal filter. For an effective and successful face rejuvenation, the combination of both, Juvederm® for the lower half of face and Botox® for the upper half of face, helps in reducing wrinkles. One of the active ingredients which are predominantly used in the Botox® Cosmetic include Clostridium botulinum, that is also known as potent poison, act as relaxing agent for the muscles. As per Swedish magazine Illustrated Vetenskap (“Science Illustrated”), Clostridium botulinum is extremely poisonous that just 500 grams of it are considered enough to kill the whole population of the world (note: in 2016, they published an article claiming this to be the most poisonous product in the world). However, the prime function of Botox initially was to treat the neuromuscular disfunctions like squint, migraine, and spasm. The properties of Botox® as a wrinkle reducing agent was discovered by sheer coincidence at the hands of Drs. Alastair and Jean Carruthers in 1987. Recognition of Botox gained strength in plastic surgery in 1990's. Allergan in 2002 received FDA approval for a less concentrated form (Botox Cosmetic) to be used for facial cosmetic treatments. Before Botox was introduced, collagen was “the substance” to be injected in order to reduce age lines and wrinkles. Biomatrix was the first company to receive a CE certification for a cosmetic HA-based filler (1995). Only one year later, Q-Med gained the same for Restylane®. This heralded the end of collagen as #1 dermal filler.
Allergan announced Juvéderm® as
Number 1 selling brand of dermal fillers in 2011. The
2013 annual report of Allergan lists sales of the Botox Cosmetics as about $912 million. The net sales of other facial aesthetic like Allergan’s Juvederm® (developed originally by French organization Corneal and in 2006 purchased by Allergan) were already US $478 million, which shows an increase of 23% compared to last years figures.
Allergan’s main competitors are
- Galderma (with US:Dysport and EU:Azzalure botulinums and after acquisition of Q-Med with Restylane® as their main dermal filler)
- Valeant (Canada) with Sculptra® for USA & Canada and (till 2014) US sales rights for Perlane® and Restylane®
- Merz (Germany) with dermal fillers Belotero® and Glytone® (sales rights purchased from Pierre Fabres, France) and US:Xeomin® / EU:Bocouture®.
Other main examples of dermal fillers based on hyaluronic acid include Teosyal®, Stylage®, Puragen®, Prevelle® Silk, Dermavisc® and Decoria®. All these products are dermal fillers and they all are based on just one project: Healon. How this happened is explained in detail in the book and in short below.
Hylaform is first dermal filler being based on hyaluronic acid. After their increasing success in the skin care market, Biomatrix decided to add viscoaugmentation to their viscosupplementation range. Viscoaugmentation as a concept involves injecting HA as dermal filler under the skin along wrinkle lines to remove or reduce them (by lifting them upwards). This already existing procedure was used with collagen so far, but incorporated various, in particular allergic side effect reactions. The need for a product surrogating collagen as anti wrinkle substance was growing steadily. This was achieved in 1984, when Balazs was successful to crosslink HA and to produce a gel (Hylan B). At the end of 1984, Ågerup joined Biomatrix as a part-time consultant and got CEO for their subsidiary in Sweden 1987. One of his first tasks was to start the clinical trials on the Hylan B. In this regard, a trial began in Germany. While initial focus was on usage of Hylan B in combination of reconstructive breast surgeries, it was shifted towards the capabilities of viscoaugmentation (filling) later. Its cosmetic nature included reduction of wrinkles and scar smoothing. Several trials in the US, Sweden and Germany restulted in the product Hylaform®.
Since Biomatrix had some funding problems at the beginning of the 1990’s, Ågerup was was working only part time at Biomatrix as CEO and used his free time to work more or less secretly for three other companies (for more interesting details please read the original book):
- Establishing Swedish company Bohus BioTech to optain HA from rooster combs, which results in (potential) competition to Synvisc and Healon
- Transfer of knowledge to Corneal how to gain HA from rooster comb
- Engineering the process how to gain hyaluronic acid for dermal fillers from bacteria via fermentation (Restylane made by Q-Med).
This transfer of the knowledge lead to new competition. Waldemar Kita was the founder of French company Corneal beginning of 1986. Being an optician Waldemar Kita focussed on specializing in the production of the intraocular lenses. It was the market segment which was growing rapidly due to development of cataract surgery markets. Corneal after some time realized unforeseen obstacle in the selling of intraocular lens. However, it was missing a necessary component for implanting intraocular lenses: hyaluronic acid, which was necessary for future growth. Most of the eye physicians prefer to purchase the hyaluronic acid and intraocular as a package. This package solution was already sold by Pharmacia, through subcontractor producing the intraocular lens. Corneal started searching for potential suppliers and found Ågerup based on the recommendation of IIan Hoffman, who was a former employee in Pharmacia and was heading Canadian Domilens subsidiary that was French producer of intraocular lens which was involved in Canadian incident.
The partnership between the Balazs and Ågerup, begun in 1984. After Ågerup signed the agreement with Bio-matrix, an Uppsala based company was started by him named as Q-Med AB.This company was significantly ineffective in its initial years, and in this regard, no remarkable activity occurred till May 1988. The ambitions of Q-Med won't be published till the release of the 1989-90 annual report. After its publication, that aim of Q-Med was basically to contract the manufacture of pharmaceuticals on a small scale for short-term purposes and clinical trials. It also announced that two essential business takeovers were made in the same period. The major result in this regard was 50% ownership of the Equinord KB and Up Will investors AB. Ågerup also expressed interest of Q-Med in the utilization of hyaluronic acid by the adhesive way. Nothing gained the interest of Q-Med, and thereby, this was not mentioned again in next three to four years. Ågerup takes control of Svenska Biomatrix as CEO, and as CEO he was involved in carrying out and organizing the clinical trials for Synvisc, Hylaform, and Gelvisc Vet. The first contact of Ågerup with cosmetic market was made because of his appointment as the CEO of Biomatrix AB. As financial problems appeared for Biomatrix, it became quite clear that the external financing was required to maintain and follow clinical trials, both that were underway as well as those that were planned for Synvisc at Swedish affiliate. Ågerup played a central role in affairs of Biomatrix but after some time, the work of Ågerup decreased in Biomatrix, regardless of the half and full-time assignments, he also had some time for various other projects and thereby he was very diligently working for about Balazs and Biomatrix and no idea. Formation of Bohus Biotech company in Stormstad had the main purpose of creating the hyaluronic acid through roosters combs for the use of pharmaceutical products based on hyaluronic acid and different surgical devices for the eye surgery. Considering the competitor of Healon and potential competitors of Synvisc. Dissemination of the knowledge related to the manufacturing of hyaluronic acid. Developing the dermal filters based on hyaluronic acid within dermal fillers within the Q-Med (Restylane) by bacterial fermentation.
One thing that Ågerup never mentioned in the meetings was the indented purpose of new form of hyaluronic acid. Though it was not quite long before Corneal understand that new product was marketed towards the cosmetic industry. When the revolutionary new product was released by Q-Med Restylane by the end of 1996 while Corneal was in search of the same market. Only after four years of Restylane of Q-Med, Corneal was also awarded for the CE certification of Hydrafill® and Juvéderm. Both of these products were based on the hyaluronic acid which was produced through bacterial fermentation. The forward march of Corneal was actually carried by Gilles Bros and team, which included Estelle Pirons among other, who later participate in the development of the dermal fillers based hyaluronic acid.
A bold claim which is made by Allergan is not verifiable, as the owner of Q-Med, Galderma, refrained everyone from presenting the sale of individual products in the annual reports of the company. It is also verifiable that the Galderma considered Restylane as one of the eighth strategic brands which sell about 100 million Euro each, and according to this annual report from 2011, when the sale of Perlane and Restylane on the American market are taken into consideration, amount grows by the US $181.6M. The market research evaluated pharma estimated global sale of Restylane during 2011 to be the US $345M.
During the legal proceedings between Q-Med and Biomatrix Ågerup, it became known that Ågerup simultaneously had been involved in the projects related to Restylane and Hylaform in Germany. He recruited same plastic surgeon for the testing of the Restylane who was previously hired by the Biomatrix in order to test the Hylaform.
Hyaluronic acid gave rise to a large number of different successful products within the different regions of application. For the successful product launch, a pre-requisite is still skillful integration and management of a multitude of the factors. This book was basically aimed at creating a better understanding of the successful development of the nine products. The nine products in this regard included Healon, Synvisc, Hylaform, Restylane, Microvisc, Viscorneal, Juvederm, Esthélis/ Belotero and Xalatan. The analytic tool in this regard selected was the extended version of the frequently used model within the premises of market analysis, in particular, 4 P's model which is also known as marketing mix. This model focus on promotion, place, price, and products. In the present analysis, three Ps to original analysis were included, i.e., Possession, Permission, and people. People in this regard refers to groups and individuals who have a considerable impact on the development of the particular product. Permission is also used in order to analyze the authority of different institutional and governmental approvals, which are essential for successful marketing and launch of the products. Possession, on the other hand, refers to the importance of the owner including ownership changes for the success of a product on the market. Type of owner primarily refers to the owner of the product, however in some situation, discussing ownership with respect to one who holds product license in a specific market.
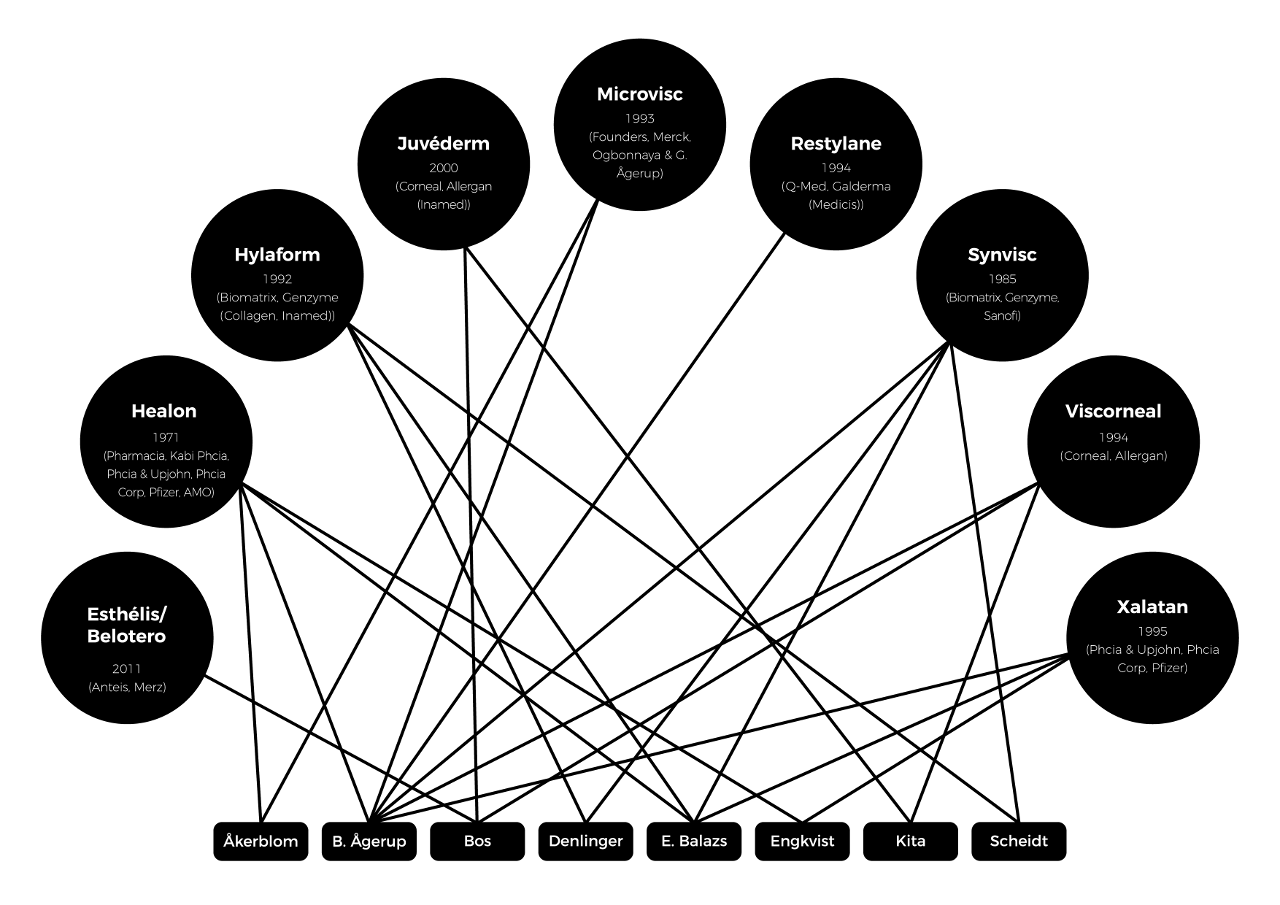
Relationship between the 9 products persons involved in more than one product.
If you found this summary interesting, I want to invide you to read the original book. You can download it here (http://liu.diva-portal.org/smash/record.jsf?pid=diva2%3A807193&dswid=4626) or get a paperback here (https://www.amazon.com/Magic-Molecule-Improved-Lives-Millions/dp/9175190761). It covers much more details including interesting background information (e.g. court cases), further cooperations/spin-offs (Anteis/Belotero, Teosyal, Glytone, Stylage, ...), conflict management and more financial figures/price strategies...
This book shows that its author, Börje Svensson, is a true insider and expert in this field and spent lots of efforts doing his research for this book. It is a very interesting book to read.
And Börje Svensson is right, hyaluronic acid really is a magic molecule.
References
Svensson, B. (2015). The Magic Molecule: that has improved the lives of millions. Linköping University Electronic Press
[End of the summary]